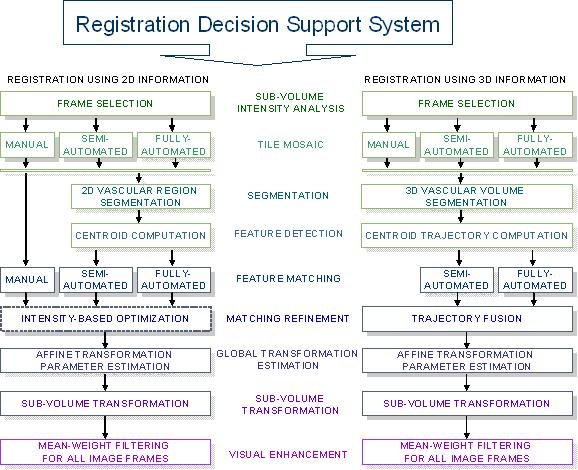
Figure 1: An overview of the three-dimensional volume reconstruction.
We present a problem of three-dimensional volume reconstruction from florescent confocal laser scanning microscopy (CLSM) imagery. We overview a three-dimensional volume reconstruction framework which consists of (a) volume reconstruction procedures using multiple automation levels, feature types, and feature dimensionalities, (b) a data-driven registration decision support system, (c) an evaluation study of registration accuracy, and (d) a novel intensity enhancement technique for 3D CLSM volumes.
The motivation for developing the framework came from the lack of 3D volume reconstruction techniques for CLSM image modality. The 3D volume reconstruction problem is challenging due to significant variations of intensity and shape of cross sectioned structures, unpredictable and inhomogeneous geometrical warping during medical specimen preparation, and an absence of external fiduciary markers. The framework addresses the problem of automation in the presence of the above challenges as they are frequently encountered during CLSM-based 3D volume reconstructions used for cell biology investigations.
The objectives of the presented three-dimensional volume reconstruction framework are summarized as follows: (1) automate alignment of sub-volumes (physical sections) from multiple cross sections, (2) obtain high resolution image frames by mosaicking (i.e., stitching together), (3) quantify the accuracy of volume reconstruction using multiple techniques, and (4) visualize the reconstructed volumes in three-dimensional environments for visual inspection and quantitative interpretation.
In our work, the three-dimensional sub-volume registration problem is viewed primarily as an alignment problem. It is approached by extracting two- or three-dimensional features from each sub-volume and registering the sub-volumes based on the analysis of detected features. We present three sets of techniques classified as pre-processing, main-processing, and post-processing techniques for 3D volume reconstruction. First, the pre-processing steps include (a) sub-volume intensity analysis for image frame selection and feature detection, (b) tile mosaicking using different automation levels and user expertise followed by accuracy evaluation, (c) 2D region or 3D volume segmentation using disk/sphere-based region/volume growing technique, and (d) feature detection based on 2D or 3D segmentation for accurate feature matching and registration alignment optimization. Second, the main-processing steps aim at achieving the most accurate sub-volume alignment, and include (a) feature matching (feature correspondence) using different levels of automation and collaborative mechanisms with web services followed by accuracy evaluations, (b) registration refinement based on different registration accuracy evaluation criteria, (c) optimal global transformation estimation, and (d) sub-volume transformation to construct a 3D volume for visualization. Finally, the volume post-processing step enhances visual saliency of the reconstructed 3D volume by minimizing distortions of the local image intensities (e.g., gradients of edges), and provides comparative results for enhancement with the existing methods using several image quality assessment metrics.
The primary contribution of this work is the presentation of a new theoretical model for three-dimensional volume reconstruction that includes reconstruction methodology, a data-driven registration decision support, automation, intensity enhancement for processing volumetric image data from fluorescent confocal laser scanning microscopes (CLSM). Researched methods have been fully implemented in the Image to Knowledge (I2K) software package developed at the National Center for Supercomputing Applications (NCSA).
The broader impact of the presented work is in providing the algorithms in a form of web-enabled tools to the medical community so that medical researchers can minimize laborious and time intensive 3D volume reconstructions using the tools and computational resources at NCSA.

Figure 1: An overview of the three-dimensional volume reconstruction.